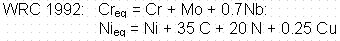WSRC-MS-2001-00544
Considerations for the Weldability of Types 304L and 316L Stainless Steels
P. S. Korinko and S. H. Malene
Westinghouse Savannah River Company
Aiken, SC 29808
This document was prepared in conjunction with work accomplished
under Contract No. DE-AC09-96SR18500 with the U.S. Department of Energy.
DISCLAIMER
This report was prepared as an account of work
sponsored by an agency of the United States Government. Neither the United States
Government nor any agency thereof, nor any of their employees, makes any warranty,
express or implied, or assumes any legal liability or responsibility for the
accuracy, completeness, or usefulness of any information, apparatus, product
or process disclosed, or represents that its use would not infringe privately
owned rights. Reference herein to any specific commercial product, process or
service by trade name, trademark, manufacturer, or otherwise does not necessarily
constitute or imply its endorsement, recommendation, or favoring by the United
States Government or any agency thereof. The views and opinions of authors expressed
herein do not necessarily state or reflect those of the United States Government
or any agency thereof.
This report has been reproduced directly from
the best available copy.
Available for sale to the public, in paper, from:
U.S. Department of Commerce, National Technical Information Service, 5285
Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax:
(703) 605-6900, email: orders@ntis.fedworld.gov online
ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department
of Energy and its contractors, in paper, from: U.S. Department of Energy, Office
of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062,
phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Summary
The susceptibility of austenitic stainless steels to solidification
cracking and lack of penetration, two distinct weld characteristics
related to the chemical composition of the base material is reviewed.
The propensity for cracking is determined primarily by the
solidification mode and the amount of residual tramp elements such as
phosphorous and sulfur. High sulfur levels can lead to weld centerline
cracking and Heat Affected Zone (HAZ) cracking while very low sulfur
levels (less than ~50 ppm) in types 304L and 316L are associated with
lack of penetration weld defects and a distinct loss in puddle control
during fusion welding. A calculated Creqivalence to Nieq
ratio of 1.52 to 1.9 is recommended to control the primary mode of
solidification and prevent solidification cracks in type 304L while the
Creq/Nieq ratio of 1.42 to 1.9 is recommended for
type 316L stainless steel. A lower limit of 50 ppm sulfur is
recommended to avoid possible lack of penetration. The ranges should be
validated by welding trials for specific weld processes and
applications.
Background
A small amount of carbon alloyed with iron makes steel.
Iron is allotropic in that it exists in at least two distinct
crystalline forms, primarily dependent upon temperature. At high
temperatures the Face Centered Cubic (FCC) crystal structure of iron is
stable and the term used to describe this phase is austenite. At very
high and low temperatures the Body Centered Cubic (BCC) structure is the
more stable phase and is given the name of delta and alpha ferrite,
respectively. Rapid cooling with the attendant high solidification
rates from the molten steel can "quench in" the normally higher
temperature phase. Chemical additions (i.e., alloying additions) can
also alter the temperature ranges where these phases are the most
stable. Nickel atoms are nominally the same size as iron atoms and
arrange themselves in the FCC structure over a large temperature range.
Therefore, substitution of nickel atoms for iron atoms has the effect
of stabilizing the austenite phase down to low temperatures. Chromium
atoms are BCC and therefore a large substitutional addition of chromium
to the steel has the effect of stabilizing the ferrite phase. Carbon
and nitrogen atoms are smaller and occupy interstitial sites between the
primary atoms in a given crystal. The unit cell structure of the
austenite phase accommodates these interstitial atoms more readily than
the unit cell structure of the ferrite phase. Therefore, carbon and
nitrogen are very strong austenite stabilizers at relatively small
volume fractions. Sulfur and phosphorus are considered trace impurity
elements, remnant from primary and secondary processing. Steel can
hence be considered an amalgamation of chemical elements within small
crystals (grains) along with their accompanying grain boundaries. The
finer the grain-size, the stronger and tougher is the steel1.
Stainless steels typically contain greater than 12 weight percent
chromium for oxidation and corrosion resistance. Chromium forms a
tenacious oxide layer on the surface of the steel, imparting a
passivation layer to provide corrosion resistance. AISI (American Iron and Steel Institute)
types 304L and 316L (L for low carbon content) stainless steels (SS)
are iron based austenitic stainless steels with the compositional ranges
shown in Table 1, depending on the specification to which it is
manufactured. These alloys have adequate Ni (8 percent minimum) to be
fully austenitic in the as-formed condition with adequate Cr (18 percent
minimum) for corrosion resistance. A loss of free or un-compounded
chromium can drastically reduce the local corrosion protection leading
to preferential intergranular attack or heat affected zone (HAZ) attack,
with a concomitant degradation of mechanical properties. Such a
condition is termed "sensitization"1. The depletion of
chromium is due to the formation, growth, and precipitation of chromium
carbide particles in the grain boundaries when and where-ever the steel
encounters temperatures in the range of about 4500C to around 8500C,
most notably in the HAZ of a weld. By simply reducing the amount of
carbon available for the chemical reaction to occur with chromium, the L
grades of stainless steel result in enhanced weld HAZ resistance to
sensitization. The effects of sensitization are a loss in corrosion
resistance due to chromium depletion and a loss of fracture toughness
due to precipitation of complex carbides within and along HAZ grain
boundaries.
In general, types 304L and 316L SS are readily weldable with common
arc processes such as; gas tungsten arc welding (GTAW), shielded metal
arc welding (SMAW), gas metal arc welding (GMAW) processes and other
techniques1. These traditional arc processes produce
moderate rates of heat input with correspondingly moderate cooling and
solidification rates and will generally produce a crack free weldment
with acceptable microstructure in typical heats of types 304L and 316L
SS. However, a full understanding of specific interactions of primary
and trace elemental constituents of the alloys that can affect the
weldability is helpful in ensuring proper material specification. This
article will address some of the chemistry effects as they relate to
welding for ASME SA 240, types 304L and 316L SS. The concepts described
in this article for ASME SA 240 type 304L, can be applied to other
specifications and types of 300 series SS.
When arc welding the 300 series stainless steels, which are fully austenitic in the as-formed condition1,
research has shown that small amounts of primary delta ferrite retained
in the weld microstructure at room temperature reduce the hot cracking
tendencies. Filler materials are designed by appropriately alloying the
iron based material to ensure a minimum amount of retained ferrite in
the completed weldment. For autogenous weldments (no filler material
added) the base metal chemistries of the components to be welded
contingent with a given proportion of dilution will determine, for a
particular cooling rate, the composition and hence the microconstituents
of the weld microstructure. The microstructure and thermal history
determine the physical properties and serviceability of the resulting
weldment.
The primary mode of solidification of the weld, be it austenite or
ferrite, is therefore important for predicting the integrity of the
proposed weld from a solidification cracking perspective. For arc
welding processes, the cracking tendency can be predicted by considering
the relative amounts of the ferrite stabilizers to the amount of
austenite stabilizers. While for fusion welding processes with either
very high cooling rates (as for the high energy density beam processes
such as laser and electron beam) or very low cooling rates (as with the
in-situ casting processes) the weld nugget morphology becomes more
important than the primary mode of weld pool solidification. The beam
processes are best suited to applications that favor very high aspect
ratio weld nuggets (high depth to width ratio). When the beam processes
are used to affect nugget morphologies similar to that of GTAW, then
the same concerns over weld hot cracking and loss of puddle control
associated with material constituents similarly apply. With high energy
beam welding (laser or electron beam) performed in the non-traditional
low aspect ratio mode, loss of puddle control can be more significant
than with the arc processes as there are no arc forces present that can
dominate the weld pool stirring mode.
The concept of nickel equivalence (Nieq) is the term used
for the cumulative effects of austenite stabilizing elements as a
weighted summation of their respective concentration levels while
chromium equivalence (Creq) is similarly the term used for
the ferrite stabilizers. The ratio of the two terms, i.e., equivalency
ratio, is usually taken as Creq/Nieq, based on the
actual chemical composition of the steels involved, and can be used as a
quantitative indicator for predicting the primary mode of
solidification for arc (fusion) welded 300 series stainless steel.
Since a small but finite amount of ferrite in the finished weldment is
desired, weld pool solidification as primary ferrite is preferred to
prevent the likelihood of encountering hot cracking during welding. The
customary material specifications used in the procurement of the
various types of 304L and 316L allow for a wide range in primary alloy
concentration levels. From a commercial economics perspective the
practical chances of obtaining a base material rich in either chromium
or nickel is small. However, with minimill feed stocks being
increasingly high in recycled content and small in relative batch sizes,
the potential for receiving a base material rich enough in alloy
content to cause a weld problem, but otherwise meeting specification, is
perceived as a real possibility. Virtually all standard specifications
in current use for the procurement of 304L and 316L allow for the
possibility of receiving material overly rich in austenite stabilizers
such that weld solidification as primary austenite could occur and lead
to hot cracking in a production weld.
Chemistry
Base metal chemistry is an especially important consideration for
situations where joints are made autogenously and where recovery from
an unacceptable weld is difficult. Specifications for typical products
used in nuclear material packages and other pressure vessel
applications, for instance, include, ASME SA 1822, ASME SA 2403, ASME SA 3124 all for 304L types, ASME SFA 5.95
for 308L type, and ASME SA 240 and ASME SA312 for 316L types. The
composition ranges of the alloys and their respective specification are
listed in Table 1. For simplicity, Creq and Nieq will be calculated for ASME SA 240 types 304L and 316L, and for ASME SFA 5.9 type 308L.
Equivalence
Many researchers6,7,8,9 have examined the composition
and post weld microstructure and have each determined an equation to
calculate the nickel and chromium equivalents. Several of the equations
are listed in Table 2 for the calculation of the Creq/Nieq
ratio. The range of composition can be used to predict the possible
solidification modes or final microstructure, depending on the
researcherís methodology, using any of the equations and the
corresponding constitution diagram, e.g., the Shaeffler6, Delong7, or Welding Research Council (WRC) 19929 constitution diagrams.
In this article, the Creq and Nieq are
calculated corresponding to the equations empirically derived from the
WRC-1992 equation and then plotted on the appropriate constitution
diagram to determine which microconstituent will initially solidify. In
general, it has been found that in the absence of phosphorus and sulfur
limit considerations, a minimum of 4 volume percent ferrite1
(Ferrite Number FN 4) and a maximum of about 21 are required to prevent
solidification cracking. At very low FN, welds solidify as primary
austenite and have significant cracking tendencies, while at very high
FN the weld will solidify fully as ferrite and exhibit some of the same
cracking tendencies as with FN less than 410. In the high FN
ranges large amounts of sulfur or phosphorus may exacerbate hot
cracking tendencies. To reiterate, alloys with ferrite contents/numbers
between 4 and 21 solidify as primary ferrite with austenite (the region
indicated by FA in Figures 3 through 5).
Based on the welding handbook11 the propensity for encountering weld solidification cracking decreases dramatically at Creq/Nieq ratios slightly less than 1.5 for equivalence determined from WRC-1992 calculations, as shown in Figure 1. This Creq/Nieq ratio lower limit is similar but not identical to that produced by Suutala, et al.12 who used the Hammar and Svenson8 (H&S) Creq and Nieq equations. The difference between the "critical" Nieq/Creq
ratios for the WRC-1992 and H&S is due to different coefficients
attached to the various elements. This effect can clearly be seen by
applying the H&S and WRC-1992 calculations to a typical heat of Type
304L SS listed in Table 1. The difference between the two calculations
is a few percent.
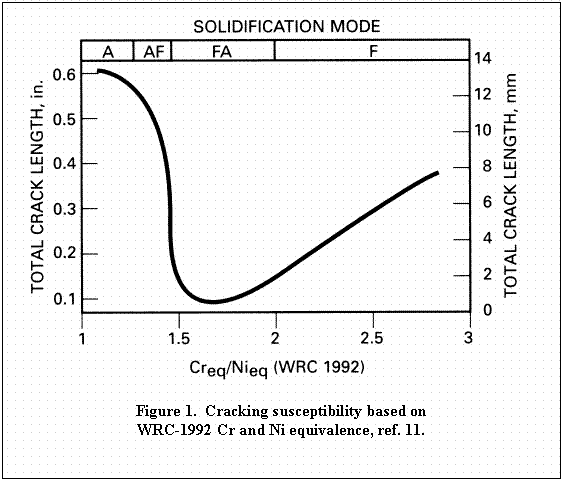
Suutala et al. report further on the effects of P and S on weld
cracking susceptibility as shown in Figure 2. Sulfur, although a tramp
element, has been shown to be beneficial in small quantities (greater
than ~50 ppm) by enhancing weld penetration. This will be discussed in
more detail later.
To evaluate the possible solidification morphologies allowed by the
specification ASME SA 240 types 304L and 316L and specification ER308L,
the appropriate composition ranges listed in Table 1 were used at the
extreme values for Ni and Cr and the Creq and Nieq were calculated using the formula from the WRC-1992 equivalence equations. The calculated Creq and Nieq values and Creq/Nieq
ratios presented in Table 4 show minimum and maximum values. The
extreme values form the corners of a rectangle when plotted on the WRC
1992 constitution diagram as shown in Figures 3 through 5.
The plot for ASME SA 240 type 304L shows that approximately one tenth
of the area (possible compositions that meet specification) enclosed by
the limit lines will solidify as austenite, and additional fourth will
solidify as primary austenite, one half will solidify as primary
ferrite, while the balance will solidify as ferrite. Similarly for ASME
SA 240 type 316L, approximately one third of the possible compositions
will solidify as austenite, one fourth as primary austenite, one third
as primary ferrite and the balance as ferrite. The solidification mode
can be altered with the addition of filler metal that is enriched in
ferrite stabilizers. Type 308L is one such alloy, itís equivalence
range is plotted in Figure 5, and it exhibits a solidification mode that
will have either primary ferrite or fully austenite. Thus by suitable
in arc alloying, dilution, the solidification mode can be modified.
It should be noted that the maximum carbon content permitted by the
ASME SA 240 was used for the calculations. In addition, due to the
absence of specification limits for nitrogen, a typical amount of 0.07%
was assumed. It has been shown that a base metal content of 0.02% N can
increase to 0.1% following a fusion weld operation due to nitrogen
pick-up from the atmosphere1 unless the weld is cooled
completely to room temperature under a protective gas shield. Certified
Material Test Reports (CMTR) can necessarily include only the measured
nitrogen content of the unwelded base material. Allowance for the
prospect of an increase in nitrogen content for the weld is made by
specifying a slightly higher Creq/Nieq in the
initial procurement recommendation. The nitrogen content taken from the
CMTR should therefore be used "as reported" in calculating the Creq/Nieq ratio. However, actual weld sample testing can be considered for critical applications.
P and S Effects
In addition to considering the solidification mode of 300 series
SS for cracking susceptibility, one must also consider the effects of
sulfur and phosphorus on weld penetration and weld cracking, as
indicated by Figure 2. Small amounts of sulfur (0.005 to 0.026 wt%)
have been associated with improved weld penetration, thus it is prudent
to specify a lower limit to better insure weldability. Descriptions of
the mechanisms are beyond the scope of this document suffice to say that
very low sulfur stainless steels (less than ~50 ppm) exhibit poor or
intermittent penetration and unstable weld pool control. Heats with
excessive sulfur content may experience heat affected zone (HAZ)
cracking or weld centerline cracking, especially in alloys rich in
austenite stabilizers. The effect of phosphorus is primarily one of
fusion zone cracking13 as opposed to weld puddle control or
penetration effects. The cracking tendencies of P and S tend to be
combined and are assumed additive as indicated by Satuula in Figure 212. The degree of cracking is largely dependent upon the solidification mode so the Creq/Nieq
ratio in conjunction with the P and S content provides some indication
of the cracking potential for a given solidification rate. This is not a
simple "rule of thumb" since Brooks et al.14 demonstrated a shift in solidification mode from primary ferrite to primary austenite for two alloys with Creq/Nieq
ratios of 1.48 and S contents of 0.04% and 0.11%, respectively. Thus
it is prudent to consider the alloy composition, application, post weld
inspection, etc., prior to specifying a possibly tighter range of
allowable compositions than permitted by the specification. In
addition, the extent of weld restraint present during welding may affect
the amount of hot cracking. For instance, the with no restraint, hot
cracks may not form even with primary austenite solidification while
with some restraint hot cracks can be a problem, as shown in Figure 6.
Other welding variables such as shielding gas composition, flux, filler
metal, etc., can have a pronounced effect on penetration and can be used
to alleviate S and P concerns therefore these considerations should be
reviewed in the context of specific applications.
Often, serviceability and machinability considerations rather than
weldability drive institutional specifications used for the manufacture
and procurement of commercial grades of stainless steel. For instance,
sulfur is added to the free machining grades of austenitic stainless
steels and from a commercial perspective it is not uncommon to encounter
heat lots of material at the high end of the specifications in S.
Conversely, minimill feedstocks are often rich in recycled content and
may contain very low residuals of P and S (<.002%, less than ~20
ppm). This situation is possible since there are no minimum
requirements in the ASTM specifications on trace element concentration
levels. A base material with an extremely low sulfur content may
produce a nearly unweldable alloy (i.e., poor penetration and puddle
control) yet one that is crack insensitive when fusion welded, as
suggested by Figure 2.
Discussion
The review presented here reveals that general material
specifications governing alloy concentration levels cover a wide range
of chemistries and are open to maximum limits concerning primary
alloying agents and minimum and maximum limits on residual elements.
The composition ranges permitted by specification ASME SA 240 for types
304L and 316L SS alone may result in alloys that promote full austenite
or primary austenite solidification modes in autogenous welds of these
materials, these solidification modes are known to be crack sensitive.
While the commercial economic realities preclude the likelihood of
obtaining alloys overly rich in either chromium or nickel, the
specifications clearly allow for the possibilities. Certainly incoming
steels have tended to be much cleaner (less tramp element content) and
more refined over the last decade. Based on the theoretically possible
rich and clean alloys allowed by specification ASME SA 240, for example,
the predicted weld morphologies suggest that during fusion welding a
potential exists for conditions that promote solidification cracks due
to primary solidification modes other than primary ferrite. Also, a
potential exists for lack of penetration and uncontrollable weld pool
conditions due to surface tension variability with very low sulfur
levels. The types 304L and 316L SS material procurement specifications
for fusion welded products should include, in addition to the
institutional specifications, high and low limits on the calculated Creq/Niea
ratio and low limits on the residual sulfur content based on CMTR data,
especially for autogenously welded products. The production weld
quality assurance enhancement promulgated through the implementation of
such chemical content calculations, based on readily available (often
required) CMTR data, represents insurance against future material
chemistry induced welding related rejects or field failures.
As an example, the composition of the "typical" ASME SA 240 type 304L SS listed in Table 1, was used to calculate the Creq and Nieq
and these data were plotted in Figure 3. It can be seen that this heat
of material will solidify as primary ferrite with a ferrite number of
approximately 9. Thus, this heat of material should not be prone to
solidification cracks due to austenitic solidification. The second
issue that is of concern is the combination of tramp elements and Creq/Nieq ratio. The Creq/Nieq
ratio for this alloy was determined to be 1.81 and the P+S was 0.05%,
Table 3. Comparing the equivalence ratio to Figure 1 indicates that
cracking susceptibility is low, and plotting this on Figure 2 also
indicates that this heat of material is not crack susceptible.
Specific Recommendation
To help insure that types 304L and 316L SS are relatively
crack insensitive, and yet weldable, particularly when welded
autogenously, limits should be placed on the Creq/Nieq ratios and sulfur content. Using the WRC-1992 constitution diagram and Creq and Nieq equations, a Creq/Nieq ratio range of 1.5 to 1.9 should be used for type 304L SS. The suggested Creq/Nieq
ratio range for type 316L SS is 1.43 to 1.9; the lower limit is
decreased due to the higher propensity for primary austenite
solidification with type 316L SS. For both cases, the sulfur range
should be 0.005 to 0.030% for acceptable penetration.
Lower S limits may be used in conjunction with lower Creq/Nieq
ratios, however, lack of penetration or the need to use filler metal
may arise. Welding trials to verify that an appropriate balance of
required properties has been achieved may also be incorporated into the
procurement specification.
Acknowledgements:
The authors would like to acknowledge John Brooks of
Sandia National Laboratories for providing micrographs and technical
input and the DOE for support under Contract No. DE-AC09-89SR18035 and
DE-AC09-96SR18500.
Table 1. Composition ranges for various specifications of types 304L, 308L, and 316L.
|
Specification
|
App
Type
|
C
Max.
|
Mn
Max.
|
P
Max.
|
S
Max.
|
Si
Max.
|
Cr
|
Ni
|
Mo
|
N
Max.
|
Fe
|
|
SA 182
(forging)
|
304L
|
0.035
|
2.00
|
0.045
|
0.030
|
1.00
|
18.00-22.00
|
8.00-13.00
|
N/A
|
N/A
|
Bal.
|
|
SA 240
(plate)
|
304L
|
0.030
|
2.00
|
0.045
|
0.030
|
0.75
|
18.00-20.00
|
8.00-12.00
|
N/A
|
0.10
|
Bal.
|
|
SA 312
(pipe)
|
304L
|
0.035
|
2.00
|
0.040
|
0.030
|
0.75
|
18.00-20.00
|
8.00-13.00
|
N/A
|
N/A
|
Bal.
|
|
SA 312
(pipe)
|
316L
|
0.035
|
2.00
|
0.04
|
0.03
|
0.75
|
16.0-
18.0
|
10.0-
15.0
|
2.00-3.00
|
N/A
|
Bal.
|
|
SA 240
(plate)
|
316L
|
0.03
|
2.00
|
0.045
|
0.03
|
0.75
|
16.00-
18.00
|
10.00-
14.00
|
2.00-3.00
|
0.1
|
Bal.
|
|
ER308L sfa5.9
|
308L
|
0.030
|
1.0-2.5
|
0.030
|
0.030
|
0.75
|
19.50-22.00
|
9.00-11.00
|
0.75
Max.
|
N/A
|
Bal.
|
|
Typical SA 240
|
304L
|
0.024
|
1.75
|
0.031
|
0.022
|
0.40
|
18.41
|
8.75
|
0.213
|
0.033
|
Bal.
|
Table 2. List of several possible Creq and Nieq that may be used.
|
Author
|
Year
|
Cr equivalent, w%
|
Ni Equivalent, w%
|
|
Schaeffler6
|
1949
|
Cr + Mo + 1.5Si + 0.5Nb
|
Ni + 0.5Mn + 30C
|
|
DeLong et al.7
|
1956
|
Cr + Mo + 1.5Si + 0.5 Nb
|
Ni + 0.5Mn + 30C + 30N
|
|
Hull10
|
1973
|
Cr + 1.21Mo + 0.48Si + 0.14Nb + 2.27V + 0.72W + 2.20Ti
+ 0.21Ta + 2.48Al
|
Ni + (0.11Mn Ė 0.0086Mn2) + 24.5 C + 14.2N
+ 0.41Co + 0.44Cu
|
|
Hammar and Svenson8
|
1979
|
Cr + 1.37Mo + 1.5Si + 2Nb + 3Ti
|
Ni + 0.31Mn + 22C + 14.2N + Cu
|
|
Siewert and Kotecki9
(WRC-1992)
|
1992
|
Cr + Mo + 0.7Nb
|
Ni + 35C + 20N + 0.25Cu
|
Table 3. Calculated equivalence values for the typical SA 240 listed
in Table 1 using both WRC 1992 and Hammar and Svenson equations.
|
WRC-1992
|
Hammar and Svenson
|
|
|
Creq
|
Nieq
|
Creq/Nieq
|
Creq
|
Nieq
|
Creq/Nieq
|
P + S
|
|
18.62
|
10.31
|
1.81
|
19.30
|
10.46
|
1.85
|
0.05
|
Table 4. Cr and Ni equivalents for the alloys shown
in Table 1 using the extremes of the composition limits as
calculated using the WRC-1992 equivalence equation shown below.
|
Specification
|
Creq
|
Nieq
|
Creq/Nieq
WRC 1992 (Min/Max)
|
|
SA 240
(304L)
|
18.00
|
9.45
|
1.20
|
|
20.00
|
15.05
|
2.12
|
|
ER308L
|
20.25
|
10.45
|
1.44
|
|
Sfa5.9
|
22.00
|
14.05
|
2.11
|
|
SA 240
(316L)
|
18.00
|
11.45
|
1.06
|
|
21.00
|
17.05
|
1.83
|
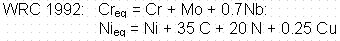
References:
- Brooks, J. A., and J. C. Lippold, "Selection of Wrought Austenitic Stainless
Steels", Metals Handbook Volume 6, Welding, Brazing, and Soldering, Metals
Park, OH, 1993.
- Anon, ASME 1998 Section II, AMSE SA-182/SA-182M, "Specification
for Forged or Rolled Alloy-Steel Pipe Flanges, Forged Fittings, and Valves and
Parts for High-Temperature Service", NY, pp. 229-249, 1998.
- Anon, ASME 1998 Section II, AMSE SA-240/SA-240M, "Specification
for Heat-Resisting Chromium and Chromium-Nickel Stainless Steel Plate, Sheet,
and Strip for Pressure Vessels," NY, pp. 363-370.i, 1998.
- Anon, ASME 1998 Section II, AMSE SA-312/SA-312M, "Specification
for Seamless and Welded Austenitic Stainless Steel Pipes", NY, pp. 477-490,
1998
- ASME 1998 Section II, AMSE SFA-5.9, "Specification
for Bare Stainless Steel Welding Electrodes and Rods", NY, pp. 203-227,
1998.
- Schaeffler, A. L. "Constitution Diagram of Stainless
Steel Weld Metal", Metal Progress, 56, pp. 680-680B, November 1949.
- DeLong, W.T., G. A. Osram, and E. R. Szumachowski, Weld
Journal, 35, pp. 521s-528s, 1956.
- Hammar, O. and U. Svensson, "Influence Of Steel Composition
on Segregation and Microstructure During Solidification of Austenitic Stainless
Steels", Solidification and Casting of Metals, The Metals Society, London,
pp. 401-410, 1979.
- Kotecki, D. J. and T. A. Siewert, "WRC-1992 Constitution
Diagram for Stainless Steel Weld Metals: A Modification of the WRC-1988 Diagram",
Welding Research Supplement, pp. 171s-177s, May 1992.
- Hull, F.C., Welding Journal, 46, pp. 399s-409s, 1967.
- Anon, Welding Handbook, Eighth Edition, Volume 4., Materials
and Applications Part 2., AWS, Miami, FL. 1998.
- Takalo, T., Suutala, N., and T. Moisio, "Austenitic Solidification
Mode in Austenitic Stainless Steel Welds", Met. Trans. A, Vol. 10A, pp. 1173-1181,
Aug. 1979.
- Li, L. and R.W. Messler, Jr., The Effect of Phosphorous
and Sulfur on the Susceptibility to Weld Hot Cracking in Austenitic Stainless
Steels", Welding Journal, 71, pp. 171s-179s. May, 1999.
- Brooks, J. A. , S. H. Goods, and C. V. Robino, "Weld
Properties of a Free Machining Stainless Steel", Sandia Report Sand2000-8002,
Sandia National Laboratories, August 2000.
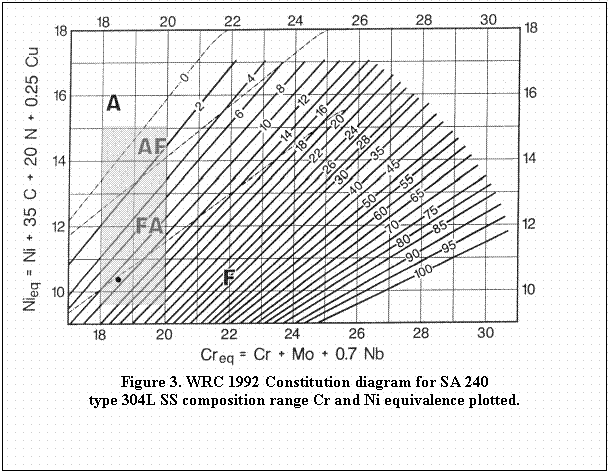
For Figures 3 through 5: Area A denotes primary solidification
as austenite with no further transformation down to room temperature (high
Nieq).
Area AF denotes primary solidification as austenite with subsequent
partial transformation to ferrite on cooling (slightly high Nieq).
Area FA denotes primary solidification as ferrite with subsequent
partial transformation to austenite (slightly high Creq) representing the
favored solidification mode.
Area F denotes primary solidification as ferrite with no further
transformation (high Creq).
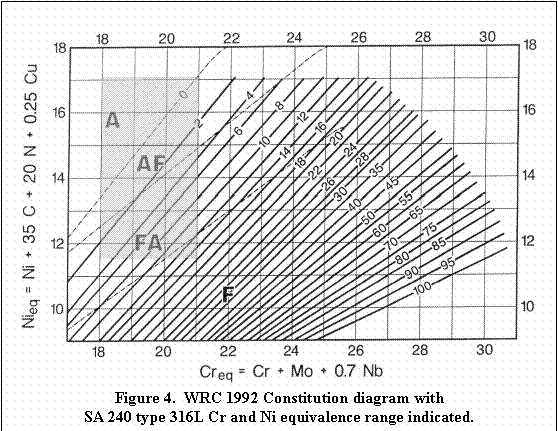
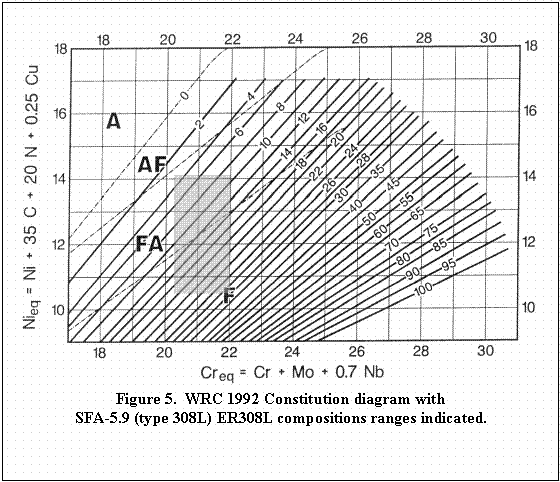

Figure 6. Micrograph showing solidification
cracks in an alloy that solidified with primary austenite.